
Supercapacitors are energy storage devices meant for applications that require high power, long lifetime, reliability, fast charge and discharge, and safety.
Unlike batteries, which store energy through chemical reactions, supercapacitors store energy electrostatically on the surface of electrodes. This enables them to charge and discharge rapidly, delivering high power density and enduring millions of charge-discharge cycles without significant degradation. However, they have a lower energy density compared to batteries, meaning they store less energy overall.
Can I use supercapacitors instead of batteries?
Some of the most common questions are about supercapacitors replacing batteries, or the differences between the two technologies. People read about the positives of supercapacitors: high power, fast charging, millions of lifecycles, excellent temperature tolerance and reliability, safety, and so forth, and want to understand whether supercapacitors could simply replace the batteries in cars, cell phones, and for example overnight storage for solar energy.
The short answer is that supercapacitors can’t replace batteries in most applications, just as batteries usually can’t replace supercapacitors, but why? The answer is most often about either the lack of energy or the lack of power.
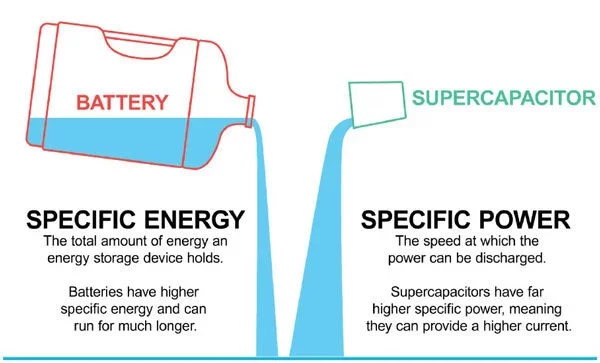
In the image above, you see the battery as a large water container. The water stored in the container illustrates the amount of energy that can be stored in a battery. Compare that to the amount of energy that can be stored in a cup, the supercapacitor in this example, and you understand energy.
Now, compare how fast you could empty and refill the cup compared to the water container (provided you have a fast-flowing water source), and you have an example for power (higher current).
What is the composition of supercapacitors?
Supercapacitors are deceptively simple devices when it comes to the components and materials but despite the relatively simple construction, developing, designing, and manufacturing supercapacitors is challenging, as each component and raw material has an enormous impact on the result: the voltage, capacitance, resistance, reliability, and every other important feature of a supercapacitor.
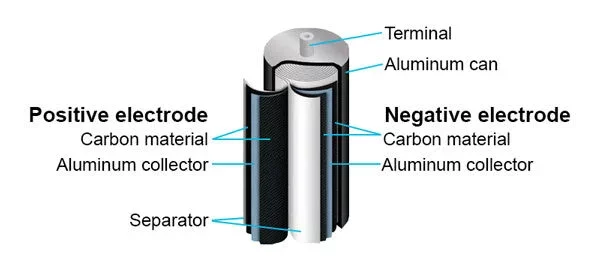
Supercapacitors have a positive and negative electrode, with an aluminum collector and separator inside an aluminum can. In addition, supercapacitors have an electrolyte, which facilitates ion movement between the electrodes, impacting voltage range and stability.
Types of common electrolytes include aqueous electrolytes (for example sulfuric acid (H₂SO₄) or potassium hydroxide (KOH), which offer high conductivity but a limited voltage range), organic electrolytes (such as acetonitrile or propylene carbonate, which provide a higher voltage window at 2.7V or higher), and ionic liquids (advanced electrolytes for high-voltage and high-temperature applications).
Supercapacitor electrodes are made out of highly porous, conductive materials. The larger the surface area of the electrode available, the better. Typically supercapacitor manufacturers use organic carbon materials such as charred coconuts, with Skeleton being the only supercapacitor company on the market with there own, synthetic carbon raw material, Curved Graphene.
The separator is a thin, porous membrane between the positive and negative electrodes to prevent short circuits. Commonly used separator materials include polymer films, and cellulose or non-woven materials.
The purpose of the current collector is to connect the electrodes to the external circuit, and they must be highly conductive and resistant to corrosion, which is why aluminum and copper foils are popular choices. At times, also carbon-based collectors are used.
What is the size of a supercapacitor?
The most common form factor for “large” supercapacitors is the D60 (60mm cell diameter) cell, but there are numerous options for even a large number of use cases. The large cells are used as building blocks for supercapacitor modules and systems, mostly used in high-power applications across power grids, industry, and transportation. An example is Skeleton’s SkelMod 162V 62F supercapacitor module, which is built using the SkelCap SCF3400 supercapacitor cell, featuring the D60 form factor.
Smaller, D33 (33mm cell diameter) supercapacitor cells are popular in the automotive and AI data center industries. Even smaller supercapacitors are found in consumer electronics, wearables, and IoT devices, among many, many other applications. For instance, SkelCap SCA0300 EVO supercapacitor cells are specifically designed for AI data center peak shaving and automotive applications.
When to use supercapacitors instead of batteries?
To use a practical example, a standard lithium-ion battery that powers your cell phone is a much better choice for that specific application than a supercapacitor because a li-ion battery can provide a full day of power for a cell phone and charge throughout the night.
So, what’s a good real-life example for a supercapacitor use case? Generally, there are not many consumer-focused applications for supercapacitors, at least in the sense that consumers would purchase supercapacitors and install or change supercapacitors themselves similar to batteries in a TV remote. Small supercapacitors are components in various devices and machinery and an everyday user is generally unaware of them.
In many ways, the same is true also for larger supercapacitors, the types that Skeleton manufactures. There are passenger cars, trains, trams, trucks, buses, and other vehicles around the world with Skeleton’s supercapacitors onboard. Similarly, industrial manufacturing facilities, power grids, intralogistics robots, port cranes, and many other applications around the globe utilize Skeleton’s solutions.
The question therefore is, when is a supercapacitor a better choice for energy storage than batteries, flywheels, or some other technology?
The image below shows the footprint comparison between standard supercapacitor energy storage cabinets, LFP (Lithium Iron Phosphate batteries, commonly used in electric vehicles, buses, energy storage systems for renewables, and as backup power) battery cabinets, and LTO (Lithium Titanate batteries, commonly used in buses, trains, and power grid stabilization applications) battery cabinets.
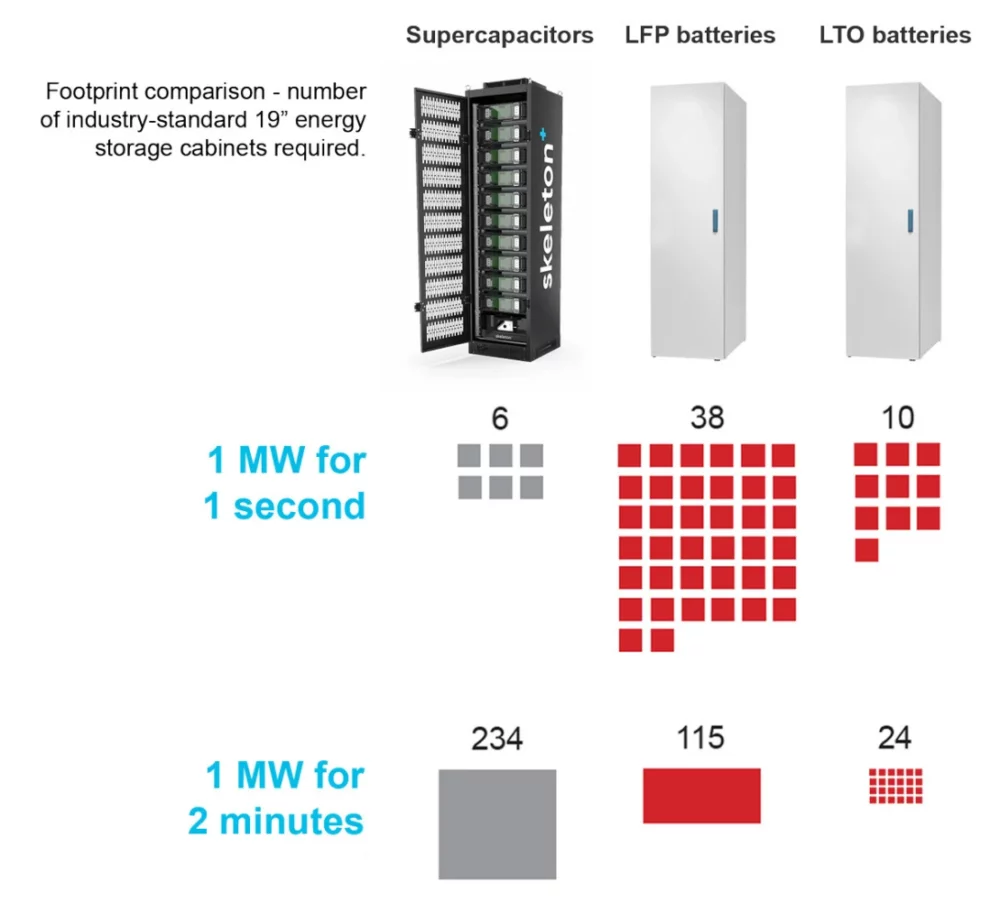
To power an application at 1 Megawatt of power for 1 second, supercapacitors are easily the most efficient solution in terms of footprint, but if the application time extends to 2 minutes, both LFP- and LTO-based solutions have an advantage. This example highlights the extreme power capabilities of supercapacitor energy storage, but at the same time, longer-term power needs are more suitable for batteries.
The key is to understand that the two technologies, supercapacitors and batteries, are not usually competing to power the same application, but are complementary technologies, each suited to specific use cases or to work together in a hybrid solution.
If you have any question or if you want additional information, please contact Nijkerk Electronics!
